![The fall armyworm Spodoptera frugiperda is one of the most important maize pests in North and South America. The name “armyworm” refers to the sudden, massive occurrence of the pest insects that attack crop fields like an entire army once their previous food supply is exhausted. [less] © Anna Schroll](http://scimondo.org/wp-content/uploads/2014/12/Heerwurm-300x199.jpg)
The fall armyworm Spodoptera frugiperda is one of the most important maize pests in North and South America. The name “armyworm” refers to the sudden, massive occurrence of the pest insects that attack crop fields like an entire army once their previous food supply is exhausted. [less]
© Anna Schroll
Plants usually defend themselves against insect feeding by producing toxins or deterrents. However, many insects have become adapted to plant defences and can feed on plant tissues containing toxins or deterrents without the expected negative effects. Insects overcome plant defences by the rapid excretion, sequestration or detoxification of toxic substances. Not only have such adaptations contributed to the vast diversification of insects in the course of evolution, they also support the success of agricultural pests specialized on certain crop plants that jeopardize crop yields every year.
With the abundance of maize grown throughout the world, it is not surprising that the crop has many insect pests, including larvae of the genus Spodoptera. In North and South America, the fall armyworm Spodoptera frugiperda is an important maize pest causing considerable damage. Like all cereals and other members of the grass family, maize plants defend themselves with chemistry. Leaves of young maize plants contain large amounts of a benzoxazinoid called (2R)-DIMBOA-glucoside. The plant also produces an enzyme active in caterpillar guts which cleaves DIMBOA-glucoside to release the sugar. The free DIMBOA formed as a result causes many insects to die or cease growing, but not the fall armyworm.
A group of researchers led by Daniel Giddings Vassão and Jonathan Gershenzon from the Department of Biochemistry at the Max Planck Institute for Chemical Ecology in Jena, Germany, have recently discovered a previously unknown detoxification strategy in these pest insects. Caterpillars of the fall armyworm and two other Spodoptera species deploy a gut enzyme that catalyzes the attachment of a sugar to the toxic free DIMBOA. The sugar group is reattached in a mirror-image orientation (forming a (2S)-DIMBOA-glucoside) so that the plant enzyme cannot remove it a second time. The scientists found out about this clever mechanism when they analysed larval frass. Modern and highly sensitive mass spectrometry and nuclear magnetic resonance spectroscopy methods showed that the benzoxazinoid identified in the larval frass was no longer identical to the substance in the plant leaves.
“We were surprised that the difference between the insect metabolite and the original plant compound was simply the stereochemical configuration of one atom. The crucial change, however, is the fact that the insect metabolite is no longer reactive to the plant enzyme and the toxic DIMBOA cannot be formed any more. The elegance of such a mechanism comes from its simplicity, yet it saves the insects from being poisoned,“ said Felipe Wouters, who performed the experiments for his doctoral thesis at the institute, summarizing the results.
Like his colleague Daniel Giddings Vassão, Felipe Wouters is from Brazil, where fall armyworm caused major losses of the maize yield before Bt maize was introduced. According to a Reuters report this summer Brazilian farmers are complaining that Bt is not protecting the plants against the fall armyworm any longer. The increasing resistance of pest insects to Bt is another reason to look deeper into the natural insect adaptations against plant defences. “If we can better understand how much this gut enzyme has helped the fall armyworm to become such a dangerous pest on maize, we may be able to use this to our advantage by impairing this insect enzyme and restoring the full defensive potential of maize against these pests,” says Daniel Giddings Vassão.
The Max Planck scientists now want to identify the enzymes and the encoding genes that are responsible for the detoxification process in the fall armyworm. They also want to look for equivalent enzymes in related species and compare these. DIMBOA is only one member of the vast variety of toxic benzoxazinoids found in grasses. If the researchers can obtain a more comprehensive picture of how benzoxazinoids are metabolized in pest insects, they may be able to design better strategies to reduce pest damage.
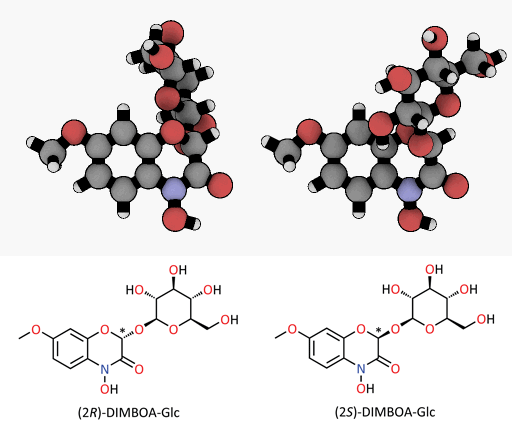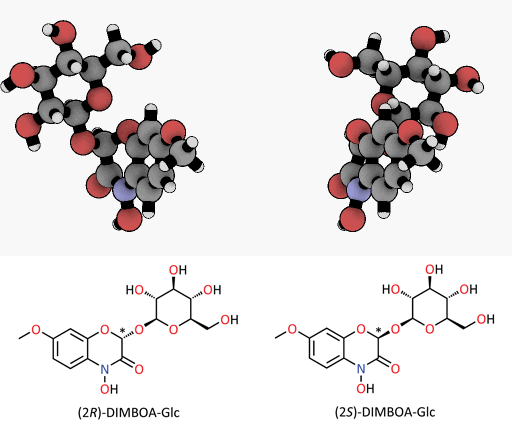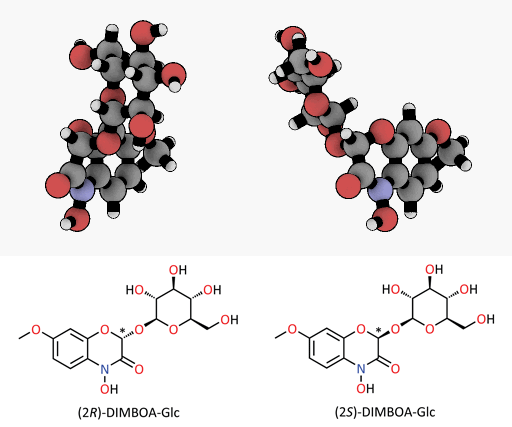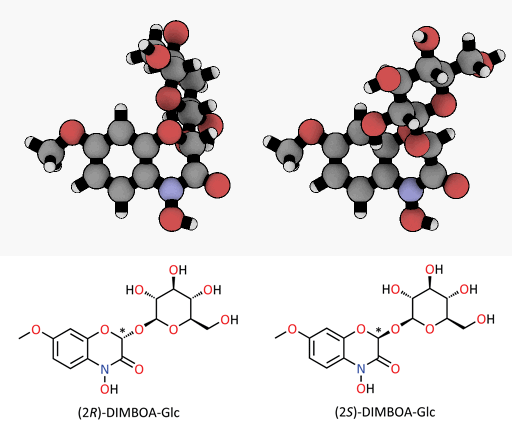
A tiny difference with a great impact: spatial representation of the epimers of the DIMBOA-glucoside. Only the (2R)-DIMBOA-glucoside (left) has a toxic effect on insects. The asterisk marks the chiral center where the configuration changes.
© MPI for Chemical Ecology/ Felipe Wouters
Research News of the Max-Planck-Gesellschaft, September 26, 2014
Original Publication:
Wouters, F.C., Reichelt, M., Glauser, G., Bauer, E., Erb, M., Gershenzon, J., Vassão, D.G. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Chemie − International Edition. DOI: 10.1002/anie.201406643
